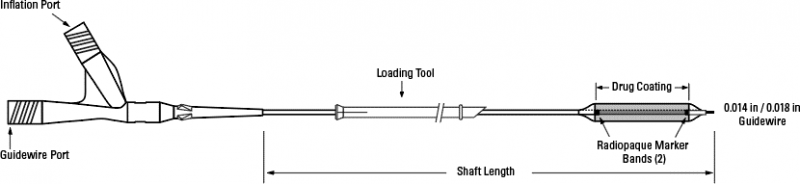
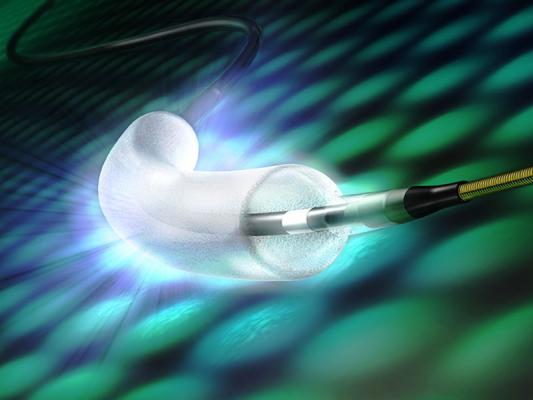
Concept Medical received IDE approval to investigate safety and efficacy of its MagicTouch Sirolimus Coated Balloon Catheter for the treatment of small coronary artery disease | tctmd.com

FDA Approves Bard's Lutonix 035 DCB For Dysfunctional AV Fistulae Treatment | Medical Product Outsourcing

Chinese FDA approve Restore drug-coated balloon for in-stent restenosis and small vessel disease – BIBA Medtech Insights

Surmodics Provides Regulatory Update Related to its FDA Premarket Approval Application for the SurVeil™ Drug-Coated Balloon | Business Wire

FDA Approves Medtronic's 200 & 250mm IN.PACT Admiral Drug Coated Balloons | Medical Product Outsourcing

FDA deems Orchestra BioMed's sirolimus-eluting balloon a breakthrough device for coronary restenosis | Fierce Biotech




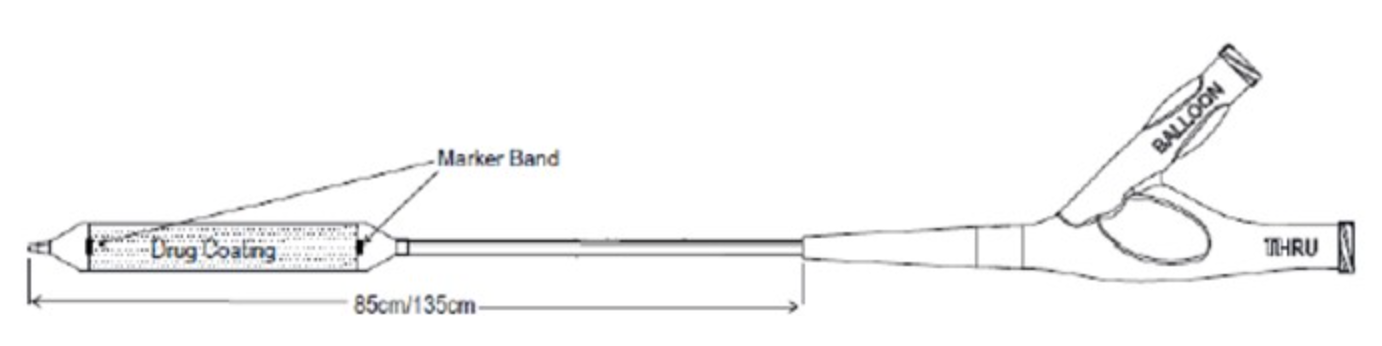





_1653668541.jpg)
