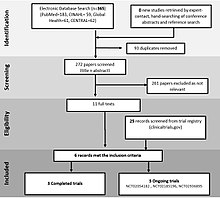
Cureus | Arginine for the Treatment of Mitochondrial Encephalopathy, Lactic Acidosis, and Stroke-Like Episodes: A Systematic Review | Media

Preferred reporting items for systematic reviews and meta-analyses: the... | Download Scientific Diagram
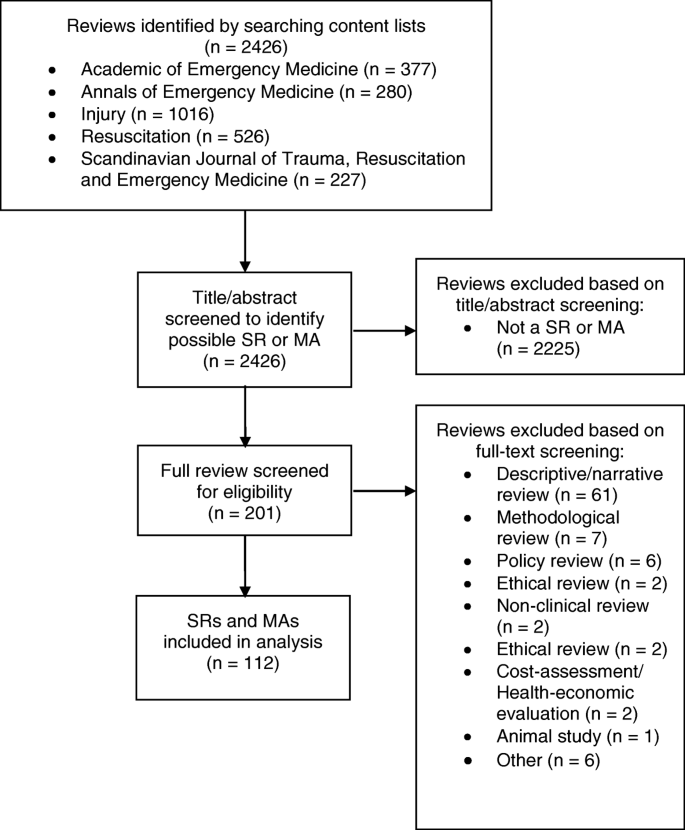
Quality of reporting of systematic reviews and meta-analyses in emergency medicine based on the PRISMA statement | BMC Emergency Medicine | Full Text
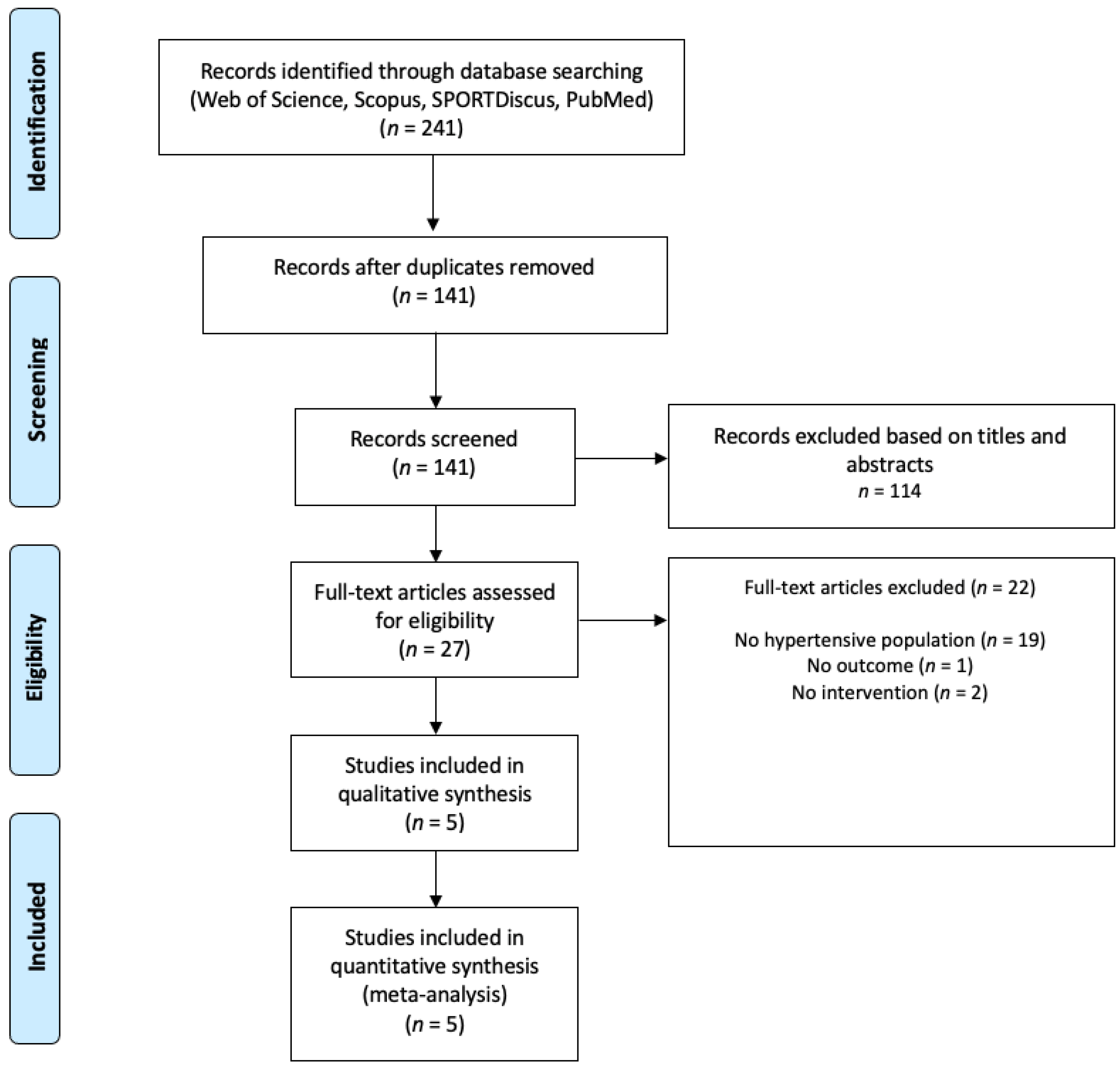
Healthcare | Free Full-Text | Effects of the Small-Sided Soccer Games on Blood Pressure in Untrained Hypertensive Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement | PLOS Medicine
![PDF] Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. | Semantic Scholar PDF] Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/e459a252789f397d7bb67a83276024f0254c682a/3-Table1-1.png)
PDF] Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. | Semantic Scholar

The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration - ScienceDirect
Appendix H: Preferred Reporting Items for Systematic Reviews and Meta- Analyses (PRISMA) Checklist | Finding What Works in Health Care: Standards for Systematic Reviews |The National Academies Press
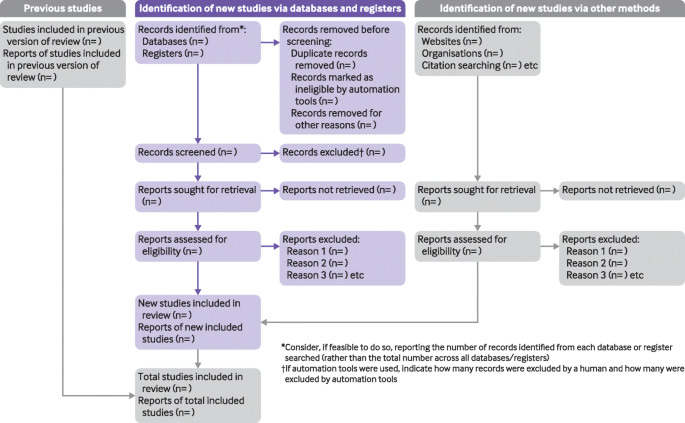
The PRISMA 2020 statement: an updated guideline for reporting systematic reviews | Systematic Reviews | Full Text

Compliance With Preferred Reporting Items for Systematic Review and Meta-Analysis Individual Participant Data Statement for Meta-Analyses Published for Stroke Studies | Stroke
![PDF] Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. | Semantic Scholar PDF] Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/acaed32791ba1befe97307b5fb1281102feb1577/4-Figure1-1.png)
PDF] Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. | Semantic Scholar
Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement | PLOS Medicine